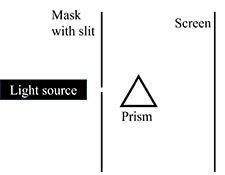
Spectroscopy
Variant i Interactive tutorial lecture
This tutorial helps students relate spectral lines, photon energies, and the electron energy levels of hydrogen.
Topics Modern and quantum / Modern physics: representations, discrete spectra, electron energy levels, frequency & wavelength, light (or shadow), photon energy, and spectroscopy
Materials
Materials by the UW team
- Clicker Questions Only

- Instructor Guide

- Pretest


- Pretest for LMS


- Exam Questions


- Equipment List

Tutorial details
Section I: Discrete spectra
Question A presents a student dialogue discussing the spectrum from a hydrogen lamp. Questions B-D guide students through calculating photon energies and relating them to differences in electron energy levels. They then revisit the student dialogue from the start of the tutorial. The Balmer series is briefly discussed.
Section II: Pattern of lines in discrete spectra
Students begin by calculating energy ratios for the electron energy levels and devise a relationship between $E_1$ and $E_n$. Students consider the limits of the line spectrum on both the right and left side. They then determine the energy transition corresponding to a given wavelength and find the location of the associated line on the spectrum.
In Questions H-I, students consider a line spectrum from an unknown gas. They determine the minimum number of energy levels to produce the given spectrum and sketch an energy level diagram that is consistent with the line spectrum.
For instruction tips, login or register as a verified educator to see the Instructor Guide.
Prerequisites
Students need to be familiar with the energy of a photon.
Coming Soon! We hope to release the discussion section on each tutorial soon.

