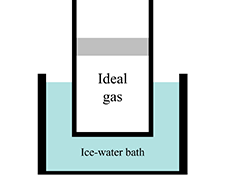
Ideal gas law
Variant i Dynamics first Other Variants Interactive tutorial lecture
Students are guiding in applying pressure, temperature, and volume to an ideal gas. A focus is on the fact that the behavior of an ideal gas is independent of the type of gas.
Topics Thermal and statistical / Thermodynamics: operational definitions, representations, atmospheric pressure, pressure, temperature, and volume
Materials
Materials by the UW team
- Instructor Guide

- Pretest

- Exam Questions


- Equipment List

Tutorial details
The first part of the tutorial leads students to recognize that a moveable piston can be used to control the pressure in a sealed container of gas. The students consider a gas sample in a cylinder sealed with a piston of mass M that can move vertically without friction. By drawing free-body diagrams for the piston they are led to recognize that the pressure is greater than atmospheric pressure by an amount determined by the mass and area of the piston.
In the second part, students apply their results from the first part to a sample of gas that is allowed to come to equilibrium first in an ice water bath and then in a boiling water bath. They are led to recognize that though the temperatures and volumes will change, the equilibrium pressures will be same because they are controlled by the mass of the moveable piston. Research has demonstrated that many students consider only two of the variables in the ideal gas law concurrently and thus will conclude that if the pressure increases, then so must the pressure. A student dialogue forces students to consider the role of the changing volume of the gas.
The third part of the tutorial provides students with practice in depicting processes on a PV diagram. The inference of the temperature of the gas from the PV coordinates is emphasized.
The fourth part of the tutorial addresses the tendency of students to attribute different properties to gases of different molecular mass. The students are led to compare two samples of different ideal gases that are at the same temperature, occupy the same volume, and are at the same pressure (which can be inferred from the masses of the movable pistons sealing their respective containers). The students are asked to compare the number of moles of each gas. (Many assume that the number will be greater in the gas of smaller molecular mass.) The students are asked whether their answer is consistent with the ideal gas law. A student dialogue helps resolve any conflicts.
For instruction tips, login or register as a verified educator to see the Instructor Guide.
Prerequisites
It is assumed that the relationship between force and pressure has been introduced. The tutorial requires students to draw free-body diagrams and draw inferences concerning the relative magnitudes of the forces exerted on an object that is at rest.
Research
- C. Kautz, P. Heron, M. Loverude, and L. McDermott, Student understanding of the ideal gas law, Part I: A macroscopic perspective, Am. J. Phys. 73 (11), 1055 (2005).
- C. Kautz, P. Heron, P. Shaffer, and L. McDermott, Student understanding of the ideal gas law, Part II: A microscopic perspective, Am. J. Phys. 73 (11), 1064 (2005).
- M. Loverude, C. Kautz, and P. Heron, Student understanding of the first law of thermodynamics: Relating work to the adiabatic compression of an ideal gas, Am. J. Phys. 70 (2), 137 (2002).
- A. D. Robertson and P. S. Shaffer, University student and K-12 teacher reasoning about the basic tenets of kinetic-molecular theory, Part I: Volume of an ideal gas, 81 (4) 303-312 (2013).
Coming Soon! We hope to release the discussion section on each tutorial soon.

